Bioquell IG-2
The Bioquell IG-2 is a fixed system that becomes integral to your equipment and operating process by providing an integrated Hydrogen Peroxide Vapour solution for removing pathogens. We work closely with you to engineer the ideal fit for your required set-up and decontamination needs.
Ideal for:
- Restricted-Access Barrier Systems (RABS)
- Filling Lines
- Cage and Rack Washers
- Isolators
- Incubators
- Freeze Dryers (Lyophilisers)
- Robotic Enclosures
- Material Airlocks
- Small-Volume Rooms

WHY CHOOSE THE Bioquell IG-2
Rapid
Validated cycles for repeatable, optimised parameters to reduce pathogens fast
Adaptable
Flexibility to create your specific decontamination solution for an enclosure or small room

Assured
Key insights and direction as well as full documentation from Bioquell’s experienced engineering and validation team
Efficient
Fast, easy operation; storage for up to 300 pre-programmed cycles

Integrated
Completely integrated solution specified to meet your equipment and process requirements
Productive
Project engineering that keeps your workflow as proficient as possible; decontamination of independent areas where applicable so you can work in an adjacent section
Applicable Solutions
CONSUMABLES & ACCESSORIES

Bioquell Hydrogen Peroxide
Bioquell’s proprietary high-purity aqueous 35% solution for use with the Bioquell IG-2 system achieves 99.9999% deactivation of pathogens and a 6-log kill with full traceability and auditing through RFID label technology

Bioquell’s proprietary high-purity aqueous 35% solution for use with the Bioquell IG-2 system achieves 99.9999% deactivation of pathogens and a 6-log kill with full traceability and auditing through RFID label technology
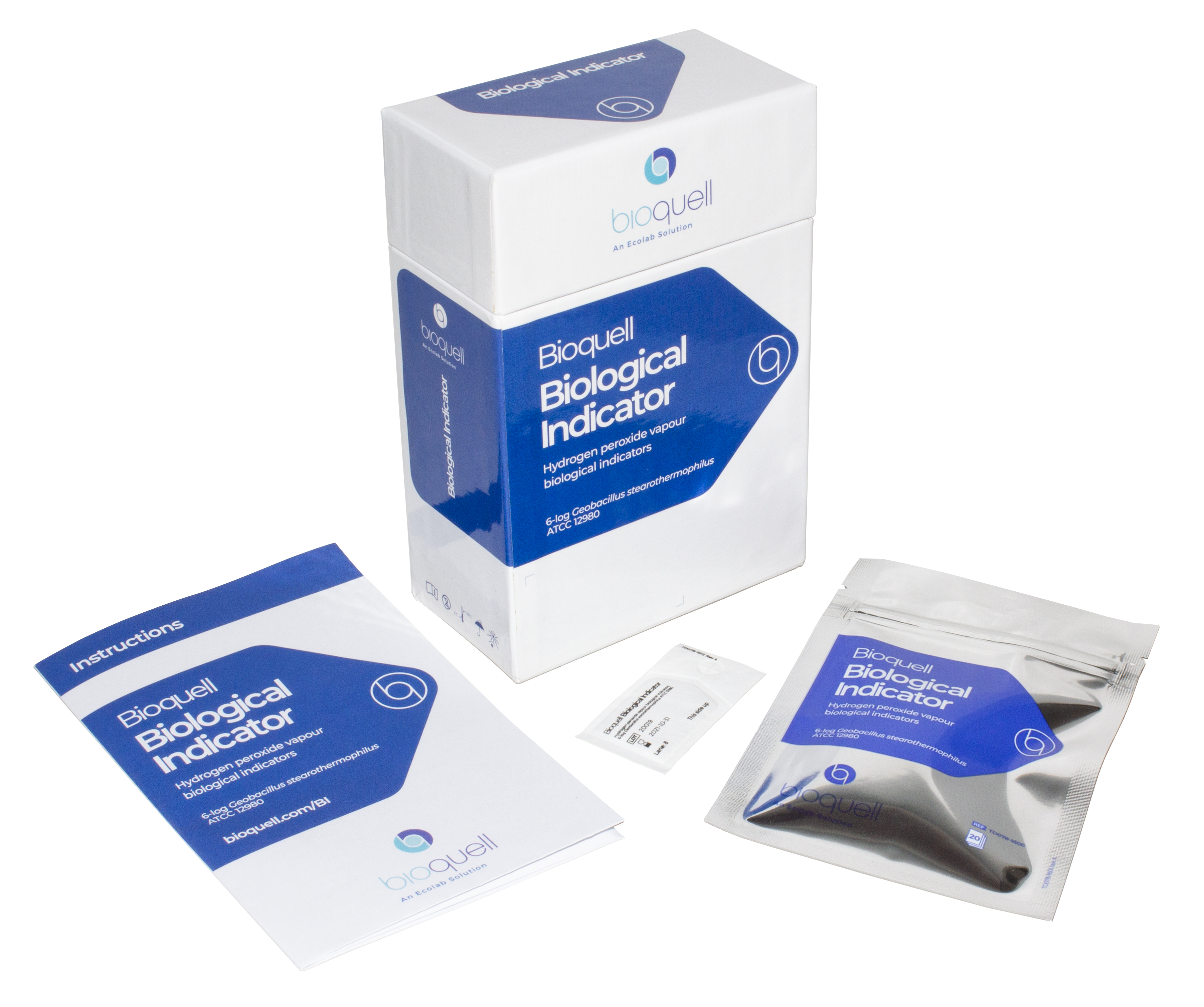
Bioquell Biological Indicators
Our individually crafted biological indicators made from Geobacillus stearothermophilus endospores ensure reliable results when seeking to validate a 6-log reduction of bioburden from the decontamination process
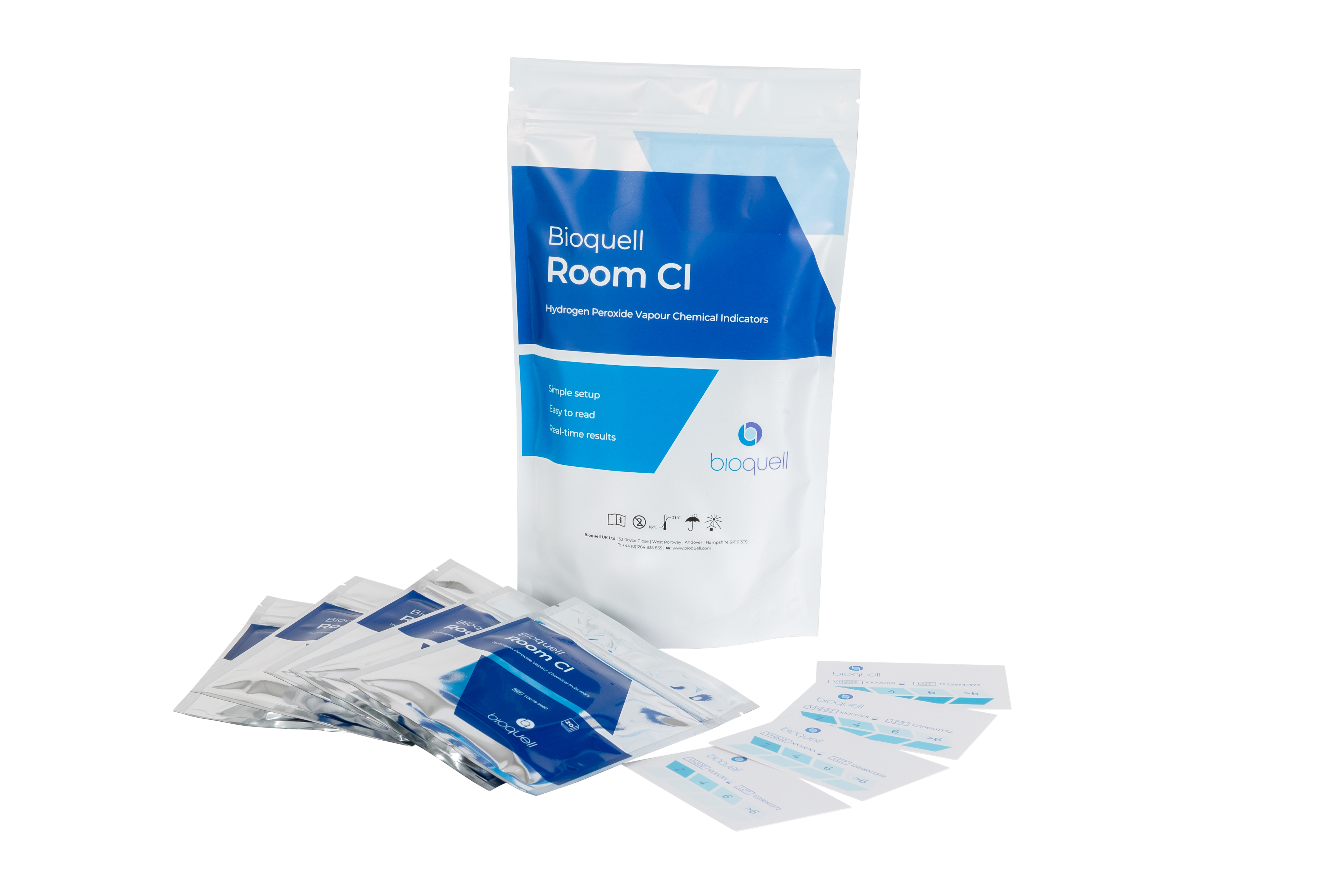
Our internally produced chemical indicators coated with our specially formulated, reactive dye provide real-time indications of your Hydrogen Peroxide Vapor decontamination cycle

The cycle end detector measures the low-level hydrogen peroxide concentration within an enclosure. It delivers a more accurate end of cycle reading than the high-level sensor in the generator. This low-level reading replaces the reading from the high-level sensor towards the end of the cycle, It is displayed on the generator screen and print out. This device is ideal for material transfer chamber or isolator applications where accurate end of cycle measurement is important to reduce cycle times. The Cycle End Detector reading must not be used as an indication for safe human entry into the bio-decontaminated enclosure. This should be done with an instrument which has been certified for this purpose.
VALIDATION
Our validation experts will assist you through each step of the process in developing the best solution for you. Bioquell offers complete validation options for your Bioquell IG-2, including for your most complex integrations.
FAQs
What will I need to do to make sure I can link this to my equipment?
The Bioquell IG-2 requires our engineers to work with you closely to fully integrate the system to your needs. Each situation varies, and we work closely with you from the start.
How do I control the system?
The Bioquell IG-2 offers a remote-control screen that can be placed at the most convenient location.
Will this offer 21-CFR Part 11 compliance?
Yes, we are able to offer an audit trail software package to support compliance with 21 CFR Part 11.
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
UK Contact
Ecolab Ltd
52 Royce Cl,
Andover SP10 3TS, UK
+44 (0)1264 835 835
bioquell.enquiries@ecolab.com










