Bioquell L-4
The Bioquell L-4 is a mobile Hydrogen Peroxide Vapour generator that can be connected to enclosures and equipment in your facility or used for room/zone decontamination.
Ideal for:
- Enclosed Systems & Equipment
- Isolators
- RABS
- Freeze Dryers (Lyophilisers)
- Bio-Safety Cabinets
- Cage & Rack Washers
- Incubators
- Filter Bank Enclosures
- Room Decontamination
Watch Video 

WHY CHOOSE THE Bioquell L-4
Rapid
Bioquell’s singular, proprietary technology for swift cycle turnarounds
Adaptable
Versatility with ability to connect to various pieces of equipment; quick and easy switch of decontamination from equipment to rooms

Assured
Thorough validation including equipment commissioning and IQ, OQ, GCD and PQ
Efficient
Fast and easy set-up; quick push-in and push-out; over 300 pre-programmed gassing cycles

Integrated
Connective capability for inclusion into more-complex systems; Modbus TCP/IP or volt-free contacts and remote start/stop for integration, automation and data capture
Productive
No need to wait for a limited temperature or humidity range to be reached as with other systems; faster start-up for more work achieved in less time
APPLICABLE SOLUTIONS
Configurations
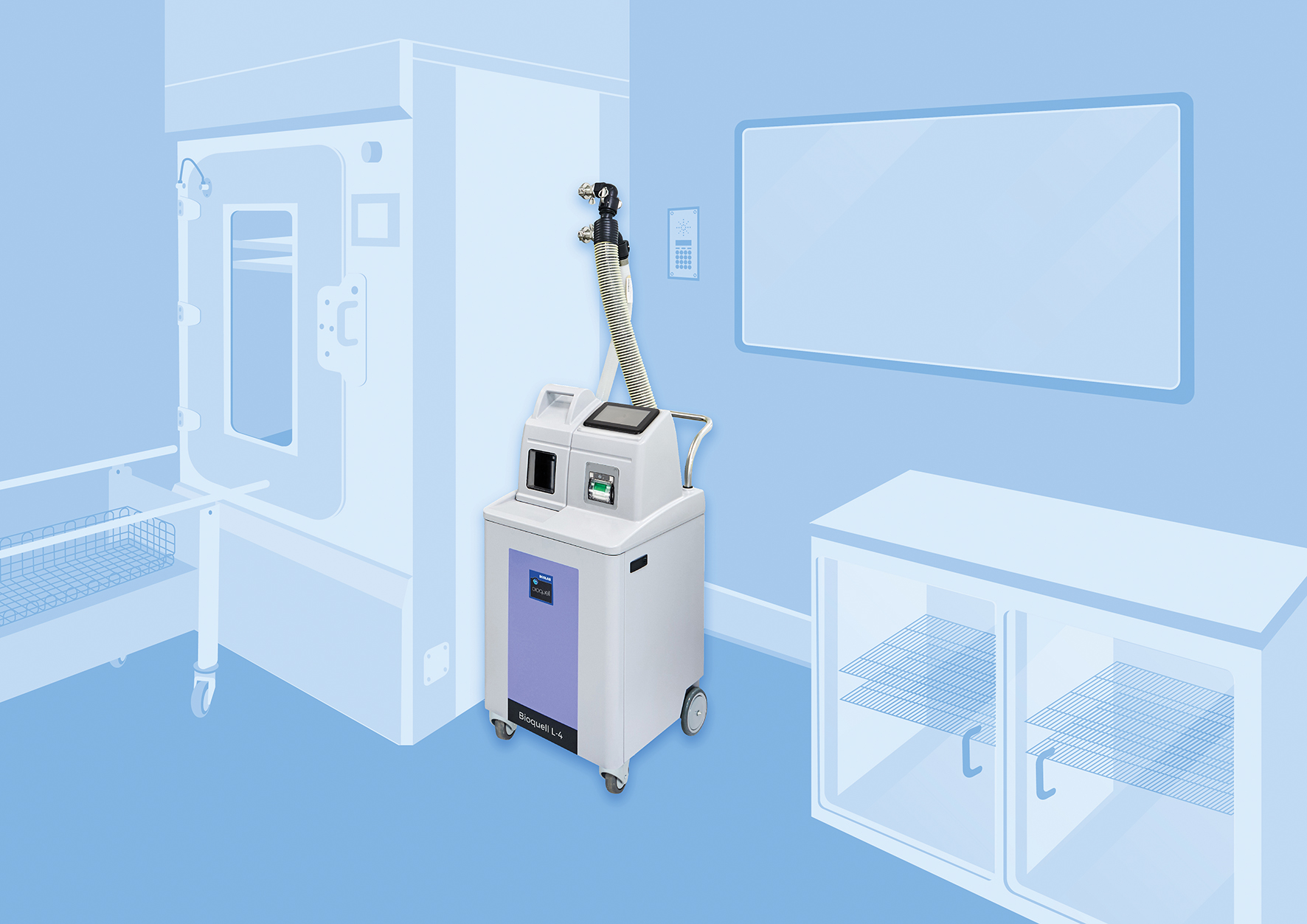
L-4 for Equipment Decontamination
Connect to equipment, isolators and enclosures with the L-4’s standard capability.
L-4 for Room Decontamination
Add Bioquell’s optional distribution head to decontaminate both equipment and small rooms.


L-4 for Filter Bank Enclosure Decontamination*
With the optional Booster fan your system gains extra capacity to decontaminate large filter bank enclosures in less time and with reduced hydrogen peroxide consumption
CONSUMABLES & ACCESSORIES

R-30 Aeration Unit
Our rapid aeration technology connects to both the Bioquell L-4 and Bioquell Z-2 to reduce cycle times by removing our Hydrogen Peroxide Vapour quickly from rooms

Our rapid aeration technology connects to both the Bioquell L-4 and Bioquell Z-2 to reduce cycle times by removing our Hydrogen Peroxide Vapour quickly from rooms

The cycle end detector measures the low-level hydrogen peroxide concentration within an enclosure. It delivers a more accurate end of cycle reading than the high-level sensor in the generator. This low-level reading replaces the reading from the high-level sensor towards the end of the cycle, It is displayed on the generator screen and print out. This device is ideal for material transfer chamber or isolator applications where accurate end of cycle measurement is important to reduce cycle times. The Cycle End Detector reading must not be used as an indication for safe human entry into the bio-decontaminated enclosure. This should be done with an instrument which has been certified for this purpose.

Give your system even greater range with this optional accessory that expands the L-4’s capabilities from decontaminating equipment and enclosures to treating a full room
VALIDATION
Validation of decontamination systems is integral to our operational DNA. Our in-house team’s seasoned engineers provide specialised commissioning, testing and proving of your Hydrogen Peroxide Vapour bio-decontamination cycles for proper performance and compliance regardless of the configuration selected or equipment to be decontaminated with the Bioquell L-4.
*Decontamination of filter bank enclosures must be verified with appropriate indicators of efficacy such as biological indicators.
FAQs
What will I need to do to make sure I can link this to my equipment?
We work with you to ensure everything you need is ready to decontaminate your equipment, from the necessary accessories to the connections required.
What is the maximum room size the Bioquell L-4 can decontaminate?
With the optional distribution head, the Bioquell L-4 could be used to decontaminate rooms up to 250 cubic meters. Contact us for more details.
When will I need a distribution head?
If you are looking to extend the capabilities of the system to decontaminate an open space, such as a room, then the distribution head would be required.
Can I connect this to my Building Management System?
Yes.
When will I need a Booster Fan?
If you are looking to decontaminate large filter bank enclosures with multiple filters systems, or single filter systems in a quicker time, then the Booster fan would be recommended.
The booster fan can also be used in small, fixed room decontamination systems to reduce cycle times
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
UK Contact
Ecolab Ltd
52 Royce Cl,
Andover SP10 3TS, UK
+44 (0)1264 835 835
bioquell.enquiries@ecolab.com











